What is Robeauté?
Robeauté is a Paris-based French medtech startup specialising in neurosurgical microrobots designed to travel through the human brain for medical intervention. Founded in 2017 by Bertrand Duplat and Joana Cartocci, the company aims to transform how neurological conditions like brain cancer, Alzheimer’s, and Parkinson’s disease are diagnosed, treated, and monitored.
In January 2025, the company secured $28 million (€27.2 million) in venture capital funding led by Plural, Cherry Ventures, and Kindred Ventures. The investment will support human clinical trials scheduled to begin in 2026, establish US operations ahead of FDA approval, and advance technology that neurosurgeons describe as potentially transformative for brain health.
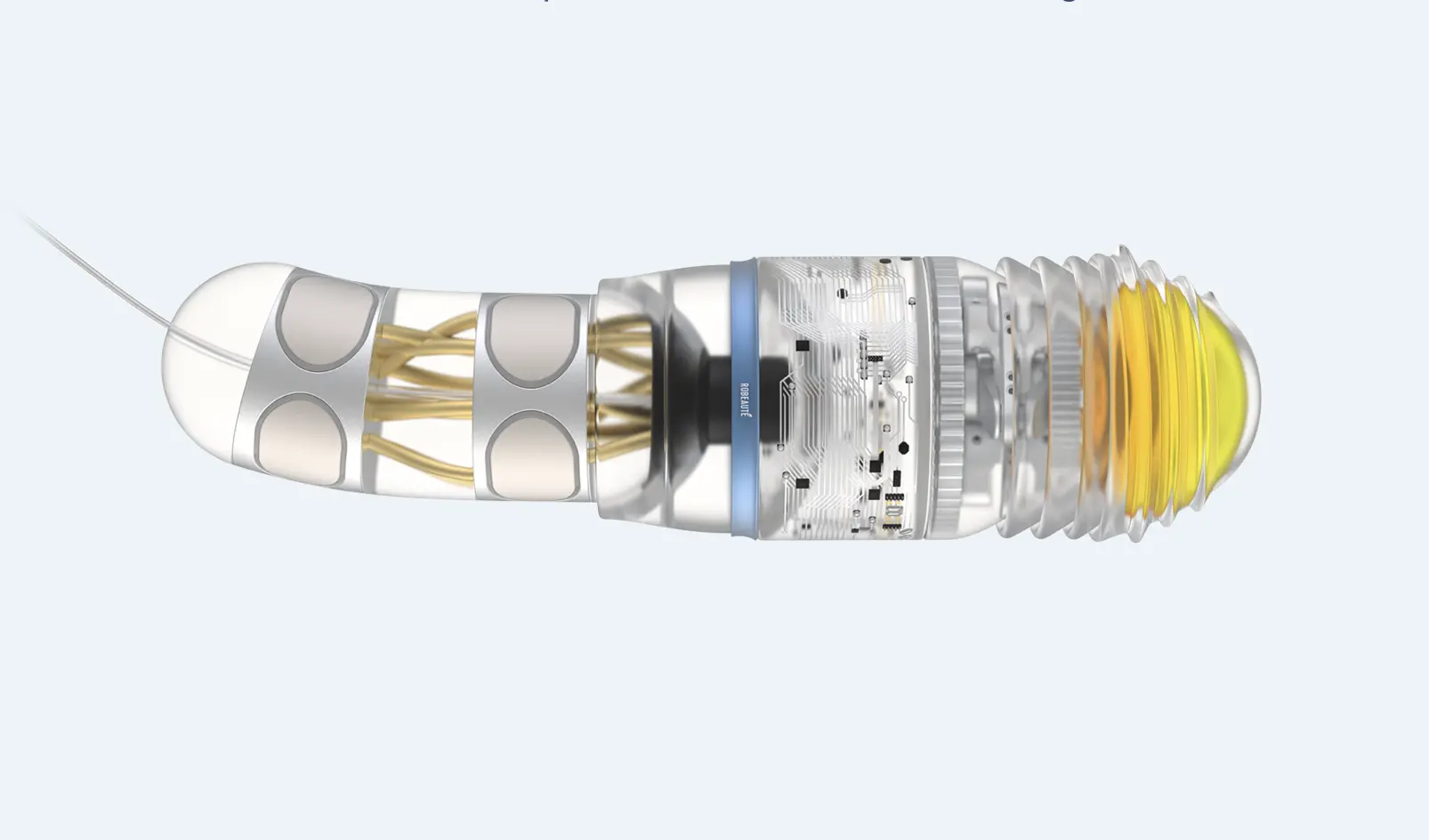
The microrobots, roughly the size of a grain of rice at just 1.8 millimetres long, can follow complex, curved paths through brain tissue to reach areas previously considered inaccessible. This breakthrough addresses fundamental limitations of traditional neurosurgery, where rigid tools pushed through the brain in straight lines restrict access and precision whilst risking damage to healthy tissue.
The Personal Story: From Tragedy to Innovation
The inspiration for Robeauté emerged from personal pain that transformed into purpose. Bertrand Duplat’s mother was diagnosed with glioblastoma, one of the most aggressive and deadliest forms of brain cancer. Despite advances in medical science, neurosurgeons could do nothing. The tumour’s location made it inoperable with conventional tools.
“It was impossible to do anything,” Duplat recalls. “The idea really came out of the pain and frustration at the inability to reach any of these meaningful areas of the brain.”
Duplat had seen the 1966 science fiction film Fantastic Voyage, which imagined shrinking people to microscopic size so they could use a tiny submarine to travel through a person’s body and repair a blood clot in an injured scientist’s brain. Decades later, with his robotics expertise and the loss of his mother, Duplat began to wonder why roboticists couldn’t actually create tiny vehicles to travel inside the body similar to the microsubmarine in the movie.
This wasn’t mere science fiction dreaming. Duplat brought over 30 years of robotics experience, including work at McGill University in Montreal and the European Space Agency (ESA), where he specialised in developing robots for extreme environments including space, radioactive sites, and the depths of the ocean. If robots could function in outer space and at crushing ocean pressures, surely they could operate inside the human brain.
More than 3 billion people worldwide live with neurological conditions, which represent the leading cause of illness and disability globally. The neurosurgery market is projected to exceed $5 billion by 2034, driven by rising neurological cases and technological progress. For Duplat, these weren’t just statistics but faces of people like his mother who deserved better options.
Record $28 Million Robeauté Funding: Backing from Europe’s Leading VCs
The Funding Round Details
In January 2025, the company announced $28 million in venture capital funding, one of the largest seed rounds for a European medtech company. The round was led by three prominent venture capital firms: Plural, Cherry Ventures, and Kindred Ventures.
Additional investors included LocalGlobe, a London-based venture capital firm backing early-stage technology companies, Think.Health, which focuses on healthcare innovation, and APEX Ventures, an existing investor demonstrating continued confidence by participating in the new round.
Particularly significant was strategic investment from Brainlab, a privately held German medical technology company and leading manufacturer of software-driven medical technology for image-guided surgery, radiotherapy, and digital operating room integration. Brainlab’s participation signals industry validation from an established player recognising the microrobots’ potential to complement their existing neurosurgery solutions.
What the Investment Means
The $28 million provides resources for several critical milestones over the next two to three years. Most importantly, funding enables human clinical trials scheduled to begin in 2026. These first-in-human studies will provide crucial safety and efficacy data required for regulatory approval whilst generating clinical evidence supporting the technology’s commercial value proposition.
The investment also supports establishing US operations ahead of FDA approval and full go-to-market efforts. The United States represents the world’s largest medical device market, and FDA approval often serves as a gold standard opening doors to other regulatory jurisdictions. By setting up US presence early, the company positions itself to move quickly once clinical data supports regulatory submissions.
Investor Perspectives
Ian Hogarth, partner at Plural, offered compelling reasoning for the investment: “Robeauté’s technology doesn’t just have the potential to transform neurosurgery; it could also fundamentally change how drug companies find the best solutions for patients. Gathering patient brain data will teach us more about diseases that are often incurable, enabling more personalised treatment.”
Hogarth draws a powerful comparison: “I strongly believe that Robeauté’s miniature robots could transform brain treatments in the same way the endoscope has transformed gastrointestinal medicine, for a part of our body that is so vital to our quality of life.” The endoscope reference provides helpful context. Before endoscopes, diagnosing and treating gastrointestinal conditions required major surgery. Endoscopes enabled minimally invasive procedures, transforming the field. Similar transformation potential exists for neurosurgery.
Filip Dames, Founding Partner at Cherry Ventures, emphasised the mission’s importance: “The challenge posed by neurodegenerative diseases is immense, touching countless lives across the globe. What Robeauté is building truly embodies the spirit of frontier technology. At the crossroads of robotics, AI, and medicine, Bertrand, Joana, and their brilliant team are pioneering a revolutionary neurosurgical microbot. Their work isn’t just advancing microrobotic surgery; it’s reshaping how we think about precision, outcomes, and the future of care for those battling these devastating conditions.”
Revolutionary Technology: How the Microrobots Work
Size and Physical Design
The microrobots measure just 1.8 millimetres in length, approximately the size of a grain of rice. This miniaturisation represents extraordinary engineering achievement, packing propulsion, steering, tracking, and communication capabilities into a device small enough to move through the brain’s extracellular matrix without causing significant tissue damage.
The robots trail an extremely fine wire behind them as they move through brain tissue. This tether serves multiple critical functions: it maintains communication with guidance and control software housed on external computers, sends data back to surgeons operating the system in real time, maintains a pathway for relaying microsurgical tools or drugs to the robot, and potentially allows tissue samples to be sent back to surgeons without the robot exiting the brain.
Curved Path Navigation
One of the biggest limitations of current neurosurgical tools is that they’re linear. Traditional probes and instruments can only access portions of the brain in a direct line from the point of entry through the skull. This restriction leaves vast regions of the brain inaccessible or reachable only by pushing instruments through intervening healthy tissue, risking serious damage.
The microrobots fundamentally solve this problem. Unlike rigid instruments, these devices can follow curved, three-dimensional routes through the brain’s extracellular matrix. Imagine trying to reach the centre of a sphere by only moving in straight lines versus being able to curve around obstacles. The curved path capability opens up brain regions that conventional tools simply cannot reach safely.
This navigation requires sophisticated software incorporating machine learning techniques that let the robot orient itself within the extracellular matrix and follow the correct path to target brain regions. Duplat compares this navigational system to a kind of three-dimensional GPS for the brain, providing real-time positioning and trajectory guidance with submillimetre precision.
Modular Mission System
The technology employs a modular architecture built on a universal robotic core with interchangeable extensions. This design philosophy enables a single robotic platform to perform multiple distinct tasks depending on clinical requirements.
Biopsy Collection: The robot can collect cell samples from tumours or diseased brain tissue for diagnostic analysis. Current methods require inserting needles or probes that may miss small tumours or fail to sample from multiple sites. The microrobots can take samples from several locations during a single procedure, providing more comprehensive diagnostic information.
Drug Delivery: Pharmaceutical treatments for brain conditions struggle to make an impact due to the blood-brain barrier, a protective membrane that prevents most substances from reaching brain tissue. The microrobots can bypass this barrier entirely, delivering therapeutic molecules directly to target locations. This local delivery enables higher drug concentrations at disease sites whilst minimising systemic side effects.
Monitoring and Data Collection: The robots can implant sensors or electrodes that monitor the brain environment in real time. This capability promises to revolutionise understanding of neurological diseases by providing granular data about chemical, electrical, and cellular changes occurring in affected brain regions over time.
AI-Planned Trajectories and Real-Time Tracking
Surgeons guide the microrobots using AI-planned trajectories that calculate optimal paths accounting for brain anatomy, tissue density, blood vessels, and the target location. The planning software models thousands of potential routes, selecting paths that minimise risk whilst maximising accessibility.
During procedures, real-time tracking monitors the robot’s position accounting for every microsecond of movement. This continuous feedback enables surgeons to make adjustments, respond to unexpected anatomical variations, and ensure the robot stays precisely on course. The combination of pre-planned routes and real-time monitoring creates a safety net, reducing procedural risk whilst maintaining the flexibility needed for individual patient anatomies.
The Founding Team: Robotics Meets Medicine
Bertrand Duplat: CEO and Robotics Veteran
Bertrand Duplat serves as co-founder and CEO, bringing over 30 years of expertise in robotics and 3D technologies as a seasoned serial entrepreneur, inventor, and innovator. Before founding the company in 2017, Duplat built an impressive career designing complex systems for extreme environments.
His early research at McGill University in Montreal sparked his passion for haptics and robotics. He later specialised in developing robots for space exploration at the European Space Agency, for undersea applications at crushing ocean pressures, and for radioactive environments where humans cannot safely work. This experience in extreme conditions proved invaluable when tackling the challenge of operating inside the human brain, another environment hostile to conventional tools.
Duplat is also a successful entrepreneur with two prior startup exits. Most notably, he founded Virtools, a 3D software company that was acquired by Dassault Systèmes, the French multinational software company best known for its CATIA design software. This entrepreneurial track record demonstrates Duplat’s ability not just to develop technology but to build companies and create commercial value.
He holds 40 patents across various robotics and 3D technology applications, reflecting a career dedicated to pushing boundaries of what machines can accomplish. His technical depth combined with business acumen positions him perfectly to lead a venture as ambitious as building medical microrobots.
Joana Cartocci: COO and Operations Leader
Joana Cartocci serves as co-founder and Chief Operating Officer, bringing a multidisciplinary background in international relations, linguistics, and global project coordination. With well over a decade in operations and fluency in six languages, she has helped drive technological innovation across healthcare, environmental sciences, and other sectors requiring complex coordination.
Cartocci’s role proves critical in translating technical vision into operational reality. Building medical devices requires coordinating regulatory affairs, quality systems, manufacturing processes, clinical trials, and commercial strategy simultaneously. Her operations expertise ensures the company maintains the discipline and systems needed to successfully bring a novel medical device through development, regulatory approval, and commercialisation.
She describes the company’s technology with a memorable metaphor: “We like to think of our microrobot as a brain gardener, that can tend to the pathological organ from within, with a standard carrier that can be adapted to fit a variety of extensions.” This accessible communication style helps neurosurgeons, investors, and patients understand the technology’s potential without getting lost in technical complexity.
Cartocci has noted that neurosurgeons have been among the company’s biggest supporters from the earliest days. “We very quickly saw the medical community response and scientific and technical community response was overwhelming and so many people opened doors for us and wanted to be part of the mission,” she said. “They recognised the need for this solution and they recognised the intellectual challenge of trying to achieve it.”
Building a World-Class Team
Beyond the co-founders, the company currently employs about 20 people, the majority of them scientists with expertise spanning robotics, neurosurgery, materials science, software engineering, and regulatory affairs. This small but highly specialised team has accomplished remarkable progress, establishing over 50 patents and advancing from concept to animal trials in eight years.
Professor validation and support from academic institutions provided crucial early credibility. Neurosurgeons immediately recognised that the technology could address limitations they face daily, leading to collaborative relationships that informed development whilst building a network of potential early adopters once regulatory approval is secured.
Eight Years of Development: From Concept to Clinical Trials
Early Validation and Breakthrough Moment
The journey from concept to viable technology required years of painstaking development. It was only in December 2021, when the company conducted its first validation tests in animal cadavers, that Duplat and the team gained confidence they could actually succeed. That moment marked a turning point when theoretical models and simulations gave way to physical proof that microrobots could move through real brain tissue as designed.
Following animal cadaver tests, the company progressed to testing in human cadavers, validating that the technology works in human brain anatomy. Most recently, live animal tests have provided crucial data about how the brain responds to the robots’ presence, whether tissue damage occurs, and how effectively the robots can perform intended tasks in living systems.
Intellectual Property Protection
Over eight years of development, the team has established more than 50 patents protecting the technology from competitors. These patents cover the microrobot’s design, propulsion system, steering mechanisms, tracking technology, modular extension system, and navigation algorithms. This intellectual property creates substantial barriers to entry, ensuring the company maintains exclusive rights to the innovations enabling microrobotic brain surgery.
The patent portfolio also provides valuable assets beyond competitive protection. Strong intellectual property makes the company more attractive to pharmaceutical partners seeking drug delivery platforms, medical device companies considering licensing arrangements, and investors evaluating long-term defensibility of the business.
Current Status: Animal Studies and Regulatory Path
The technology is currently in animal studies as a biopsy tool. This application provides the regulatory pathway of least resistance, allowing the company to demonstrate safety and efficacy for a well-defined use case before expanding to more complex applications like drug delivery and real-time monitoring.
Focusing initially on biopsy serves strategic purposes beyond regulatory efficiency. Brain biopsies represent a clear unmet need where current methods often fail to provide adequate tissue samples. Demonstrating success in this application builds confidence amongst neurosurgeons and regulators whilst generating clinical data supporting expanded indications.
Human Trials in 2026: The Next Critical Milestone
The company is currently transitioning from animal studies to human clinical trials scheduled to begin in 2026. These trials represent the most significant milestone in the company’s history, providing the first data on safety and performance in actual patients.
Initial trials will likely focus on patients with brain tumours requiring biopsy for diagnosis. These patients face procedures where current methods carry significant risk, making them appropriate candidates for testing alternative approaches. Success in these early trials could lead to expanded studies in larger patient populations and additional indications.
The trial design will carefully balance gathering meaningful data against patient safety. Early studies typically enrol small numbers of patients with close monitoring, gradually expanding if safety profiles prove acceptable. Regulatory authorities require comprehensive safety data before approving novel medical devices, particularly for applications as critical as brain surgery.
Clinical trial outcomes will determine the timeline for commercial availability. Positive results could enable regulatory submissions within 12 to 24 months after trial completion. However, any safety concerns or performance issues could require design modifications and additional studies, extending timelines significantly.
Frequently Asked Questions About Robeauté
How do the microrobots move through the brain?
The microrobots use a tiny engine and propeller system to propel themselves through the brain’s extracellular matrix. Surgeons control movement using external computers that plan trajectories and provide real-time guidance. The robots trail a fine wire maintaining communication and providing a pathway for tools or drugs. Unlike rigid instruments pushed through the brain, these devices can follow curved paths reaching areas conventional tools cannot access.
What conditions could the microrobots treat?
Initial applications focus on brain biopsies for diagnosing tumours and other pathologies. Future applications could include delivering drugs locally to treat brain cancer, Alzheimer’s disease, Parkinson’s disease, and other neurological conditions. The robots could also implant monitoring sensors providing real-time data about brain chemistry and electrical activity in patients with epilepsy, traumatic brain injuries, or neurodegenerative diseases.
When will the technology be available to patients?
Human clinical trials are scheduled to begin in 2026. If trials demonstrate safety and efficacy, regulatory approval could come within two to three years after trial completion. Commercial availability would likely begin in the United States following FDA approval, with European availability following CE Mark certification. Widespread adoption would require additional years as neurosurgeons gain experience with the technology.
Who founded the company and where is it based?
Bertrand Duplat and Joana Cartocci co-founded the company in 2017. The company is headquartered in Paris, France, with plans to establish operations in the United States ahead of FDA approval. Duplat brings 30 years of robotics experience from McGill University and the European Space Agency, plus successful entrepreneurial exits including Virtools to Dassault Systèmes. Cartocci provides operations leadership with a decade of experience coordinating complex technology projects.
How much funding has the company raised?
In January 2025, the company raised $28 million led by Plural, Cherry Ventures, and Kindred Ventures, with participation from LocalGlobe, Think.Health, APEX Ventures, and strategic investment from Brainlab. This represents one of the largest seed rounds for a European medtech startup, providing resources for human trials in 2026 and US market entry.
How safe are brain microrobots?
The microrobots are designed to travel through the extracellular matrix between brain cells rather than damaging tissue. Their small size and curved path capability enable reaching targets without pushing through healthy brain regions. Extensive testing in animal cadavers, human cadavers, and live animals has informed safety features. Human trials beginning in 2026 will provide the first data on safety in actual patients, with regulatory authorities requiring comprehensive safety demonstration before approving commercial use.
Also Read: Spanish HealthTech Pragmatech raises €650k to roll out CE-marked AI antibiotic prescribing software